Cosmetic Product Registration in Sri Lanka - Overview
The cosmetic products industry in Sri Lanka has recorded gradual growth over the past few years, with an eventual increase in the supply of cosmetic products in the market. With the rise in per capita income, urbanization, and the use of internet, consumers have become more informed about their skin types. As a result, they are embracing international trends, leading to an increased demand for locally made private label cosmetics and imported cosmetics in Sri Lanka.
In Sri Lanka, cosmetics can only be registered if the manufacturer (the foreign brand owner company) is registered with the National Medicines Regulatory Authority (NMRA). The registration of manufacturers must be carried out by a cosmetics Legal Representative (LR) in Sri Lanka, who shall act as the cosmetic registration certificate holder and as the importer.
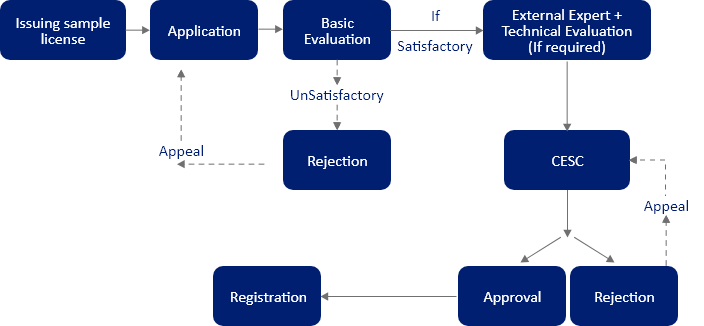
Before applying for cosmetic registration in Sri Lanka, a cosmetic import license, also known as a sample import license, needs to be obtained. Moreover, the evaluation of samples along with necessary documents is carried out by a Cosmetic Evaluation Subcommittee (CESC), appointed by the Cosmetics, Devices, and Drugs (CDD) Technical Advisory Committee (TAC).

Manufacturers aiming to place their cosmetic products in the Sri Lankan market are required to apply for a cosmetic registration certificate. In Sri Lanka, cosmetics notification is completed by submitting a cosmetic application via Form A & B, Schedule I, to the National Medicines Regulatory Authority (NMRA). Based on the inputs from the CESC, which also includes the cosmetic product safety assessment, a Provisional Registration (PR) is issued.
The PR is issued for the first year for any product falling under categories like drug, device, cosmetics, etc. If the evaluation committee deems the submitted documents satisfactory, the PR is issued. Post that, the committee issues a Full Registration (FR) for two (02) or five (05) years. Most of the time, the FR is issued for five (05) years. In case the documents are not deemed to be satisfactory or if the dossier lacks any documents, the committee can still issue a PR. However, the committee would require the applicant to submit the remaining documents along with the FR application documents. If these additional documents are also unsatisfactory, the committee can reject the cosmetic application altogether and provide reasons for the rejection.
Cosmetic Product Registration in Sri Lanka - Freyr Expertise
- Cosmetic product classification as per the NMRA cosmetics guidelines.
- Cosmetic formulation review/cosmetics ingredient review.
- Cosmetic claims review.
- Cosmetic product registration in Sri Lanka.
- Label review as per the Sri Lankan cosmetic labeling regulations/cosmetic labeling requirements.
- Sample cosmetic import license.
- Dossier compilation.
- Cosmetic registration application with the NMRA.
- Provisional Registration (PR) services.
- Full Registration (FL) services.
- Cosmetics Legal Representation (LR) and import services.
- Regulatory support services.
Cosmetic Product Registration in Sri Lanka - Freyr Advantages
- End-to-end cosmetic Regulatory consultation.
- A qualified team of experts with hands-on experience across all cosmetic categories such as skin care, hair care, baby care, oral care, beauty products, etc.
- Support in region-specific Regulatory complexities.
- Strategic local Health Authority (HA) contacts.
- A structured and cost-effective approach to ensure compliance.
- Quick turnarounds and faster time-to-market.